Solution:
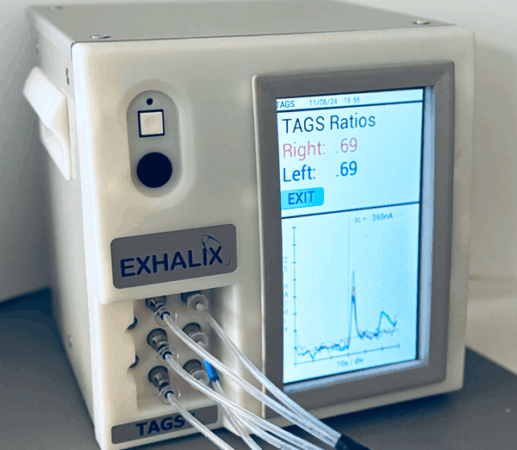
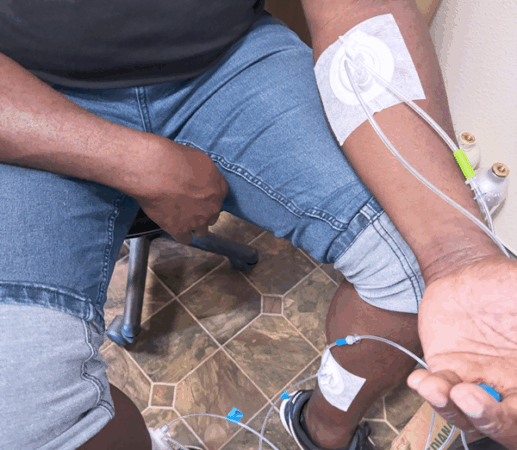
TAGS (Transdermal Arterial Gasotransmitter Sensor)
Identifies: Decreased lower limb circulation (PAD) by sensing in healthy H2S gas emissions
Predicts: Coronary artery disease or Chronic Limb Threatening Ischemia (CLTI)
Prevents: Heart attack and stroke, and diabetic amputations with early medical intervention
Better + Cheaper + Faster + Preventative
How is TAGS better than current PAD diagnostic devices?
The patented TAGS system is the first of its kind, early detection device for PAD and the only diagnostic device suitable for POC use. TAGS is better, cheaper and faster than current noninvasive PAD diagnostic devices, and the only device targeted for patient screening and monitoring.
Although TAGS is a more comfortable, accurate and easy-to-use diagnostic tool for PAD, it would not replace angiography, duplex ultrasonography, TCPO2 or ABI in every case, but it would fill a critical gap upstream: enabling early, scalable, routine detection of PAD before patients reach advanced stages requiring expensive, invasive interventions.



Meet the Team
Plus: key senior clinical, industry, and business advisors

CEO - Annette Lavoie, PhD
- Medical researcher, serial entrepreneur
- 20 years in medical device startups
- Investor relations, market strategy, exit
- Commercialization expert

CTO/Founder - Reza Shekarriz, PhD
- Mechanical engineer, sensor development
- 25 years experience product driven R&D
- Product finalization, launch strategy
- Technology inventor, Exhalix founder & CEO

COO - Heather Ferguson, PhD MBA
- Medical device quality, regulatory, operations
- 15 years experience, medical devices to market
- Product Quality System, FDA approval
- Regulator FDA expert

CMO - Ajay Nair, MBA
- Senior executive med device sales
- 20 years experience building teams
- Startup and corporations (Medtronic, Ethicon)
- Marketing, sales, and distribution expert

CSO - Nancy Kanagy, PhD
- Internationally recognized expert
- Cardiovascular physiology H2S signaling
- Professor UNM School of Medicine
- Scientific applied research expert

Clinical Testing - Ross Clark, MD
- Vascular surgeon, Assistant Professor
- UNM School of Medicine
- Clinical testing and physician adoption
- Clinical expert, H2S, PAD, and CLTI

